Endometrial Cancer Clinical Trial Pipeline Insights: 50+ Companies Shaping Future Treatment Paradigms | DelveInsight
Endometrial Cancer is a type of cancer that begins in the lining of the uterus, most commonly affecting postmenopausal women. Rising prevalence due to increasing obesity rates and aging population, along with advancements in targeted therapies and early diagnostic tools, are key factors driving market growth.
New York, USA, July 15, 2025 (GLOBE NEWSWIRE) -- Endometrial Cancer Clinical Trial Pipeline Insights: 50+ Companies Shaping Future Treatment Paradigms | DelveInsight
Endometrial Cancer is a type of cancer that begins in the lining of the uterus, most commonly affecting postmenopausal women. Rising prevalence due to increasing obesity rates and aging population, along with advancements in targeted therapies and early diagnostic tools, are key factors driving market growth.
DelveInsight’s 'Endometrial Cancer Pipeline Insight 2025' report provides comprehensive global coverage of pipeline endometrial cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the endometrial cancer pipeline domain.
Key Takeaways from the Endometrial Cancer Pipeline Report
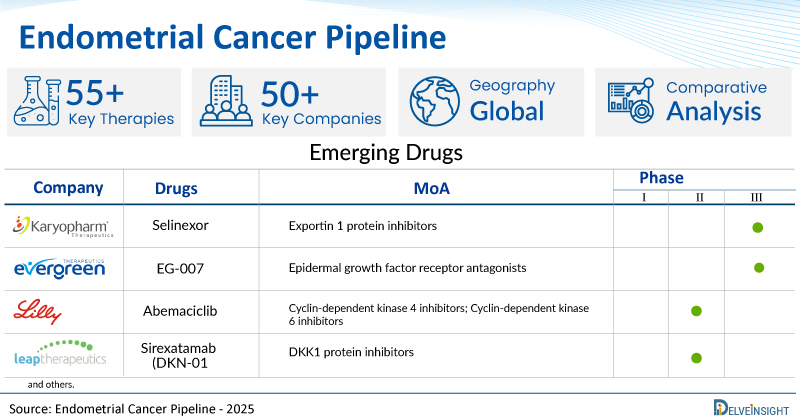
- DelveInsight’s endometrial cancer pipeline report depicts a robust space with 50+ active players working to develop 55+ pipeline endometrial cancer drugs.
- Key endometrial cancer companies such as Eli Lilly and Company, Karyopharm Therapeutics, NETRIS Pharma, TORL Biotherapeutics, Compugen, 3D Medicines, Evergreen Therapeutics, Acrivon Therapeutics, Eisai, Xadcera Biopharmaceutical (Suzhou) Co., Ltd., Huabo Biopharm Co., Ltd., Multitude Therapeutics, Context Therapeutics, Leap Therapeutics, Mersana Therapeutics, MacroGenics, Accent Therapeutics, Shanghai Henlius Biotech, Imvax, and others are evaluating new endometrial cancer drugs to improve the treatment landscape.
- Promising pipeline endometrial cancer therapies, such as Selinexor, Abemaciclib, NP137, TORL-1-23, COM 701, Envafolimab, EG-007, Prexasertib, E7386, DM002, HB0025, AMT-151, CTIM-76, Sirexatamab (DKN-01), XMT-1660, MGC026, DHX9 (ATX-559), HLX17, IEC-001, and others, are in different phases of endometrial cancer clinical trials.
- In June 2025, Daiichi Sankyo announced the first patient had been dosed in the DESTINY-Endometrial01 clinical trial evaluating Enhertu (trastuzumab deruxtecan) in combination with rilvegostomig or Merck’s Keytruda (pembrolizumab) as a first-line treatment for patients with HER2-expressing (IHC 3+/ 2+), mismatch repair proficient (pMMR) primary advanced or recurrent endometrial cancer.
- In May 2025, Kaida BioPharma announced it had entered into a manufacturing agreement with Northway Biotech, Inc., an end-to-end biologics Contract Development and Manufacturing Organization (CDMO), for the manufacturing of lead product candidate, KAD101.KAD101, a novel biologic prolactin receptor antagonist, is being initially developed for the treatment of platinum-resistant ovarian cancer (PROC) with expansion opportunities into endometrial, uterine, and breast cancers.
- In May 2025, PRISM BioLab, Co. Ltd. announced that the analysis of a combination study of E7386 created through collaboration research with Eisai Co., Ltd., and Lenvatinib mesylate will be presented by Eisai at the American Society of Clinical Oncology (ASCO) Congress 2025, held in Chicago, USA from May 30 to June 3, 2025. To determine the optimal dose of E7386 in combination with Lenvatinib in the open-label Phase Ib study (NCT04008797), expansion cohort of advanced endometrial cancer patients who progressed following platinum-based chemotherapy and anti-PD (L)1 immunotherapy have been implemented by Eisai, and the enrollment of 30 patients was completed.
- In March 2025, Faeth Therapeutics and The GOG Foundation, Inc. (GOG-F) announced that the first patient has been dosed in its Phase II combination trial of PIKTOR, which is FTH-001 (serabelisib) and FTH-003 (sapanisertib) with paclitaxel. The trial is the most advanced of its kind to investigate a novel approach of dual PI3Kɑ-mTORC1/2 inhibition targeting cancer metabolism in patients with endometrial cancer.
- In February 2025, Acrivon Therapeutics announced that the FDA had granted Breakthrough Device Designation for ACR-368 OncoSignature Assay for Endometrial Cancer.
- In December 2024, Repare Therapeutics Inc. reported positive data from its MYTHIC Phase I gynecologic expansion clinical trial evaluating the combination of lunresertib and camonsertib (Lunre+Camo) at the recommended Phase II dose (RP2D) in patients with endometrial cancer and platinum-resistant ovarian cancer (PROC) harboring lunre-sensitizing biomarkers.
- In December 2024, IDEAYA Biosciences, Inc. announced that it has dosed the first patient in the IDEAYA-sponsored Phase I trial evaluating the combination of IDE161, the company's investigational, potential first-in-class, small molecule poly (ADP-ribose) glycohydrolase, or PARG, inhibitor, in combination with Merck's (known as MSD outside of the US and Canada) anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), in endometrial cancer patients with high microsatellite instability (MSI-high) and microsatellite stable(MSS).
Request a sample and discover the recent advances in endometrial cancer drugs @ Endometrial Cancer Pipeline Report
The endometrial cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage endometrial cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the endometrial cancer clinical trial landscape.
Endometrial Cancer Overview
Endometrial cancer, which arises from the uterus’s inner lining (the endometrium), is the most common gynecologic cancer in developed countries, with its occurrence steadily increasing. This rise is linked to factors like aging populations and growing obesity rates. Most diagnoses occur in postmenopausal women, with abnormal uterine bleeding being the most frequent symptom. Because this symptom often prompts early medical evaluation, many cases are detected at an initial stage, improving treatment success.
Several risk factors play a role in the development of endometrial cancer. Obesity, metabolic syndrome, and diabetes are key contributors, with obesity responsible for about 40% of cases. Reproductive history, such as early onset of menstruation, delayed menopause, never having given birth, and infertility, also raises risk. Genetic factors, especially Lynch syndrome, increase susceptibility. In contrast, combined oral contraceptives have been linked to a decreased risk.
Diagnosis usually involves an endometrial biopsy, often following a transvaginal ultrasound to measure endometrial thickness. However, research suggests transvaginal ultrasound can produce false-negative results, particularly in Black women, indicating that biopsy remains the definitive diagnostic method. Surgical staging, based on the International Federation of Gynecology and Obstetrics (FIGO) criteria, is essential for assessing disease extent and planning treatment.
Treatment varies by stage. Early-stage disease is generally treated with a total hysterectomy and removal of both ovaries and fallopian tubes. Additional therapies such as radiation or chemotherapy may be recommended depending on risk factors. For advanced or recurrent disease, newer treatments like immunotherapy show promise. In particular, the combination of dostarlimab (JEMPERLI) with chemotherapy has improved survival in advanced or recurrent cases. Research continues to evolve to optimize these treatment approaches and improve patient outcomes.
Find out more about endometrial cancer drugs @ Endometrial Cancer Treatment
A snapshot of the Pipeline Endometrial Cancer Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Selinexor | Karyopharm Therapeutics | III | Exportin 1 protein inhibitors | Oral |
| EG-007 | Evergreen Therapeutics | III | Epidermal growth factor receptor antagonists | Injectable |
| Abemaciclib | Eli Lilly and Company | II | Cyclin-dependent kinase 4 inhibitors; Cyclin-dependent kinase 6 inhibitors | Oral |
| Envafolimab | Alphamab Oncology/Ascletis/3D Medicine | II | Antibody-dependent cell cytotoxicity; Programmed cell death-1 ligand-1 inhibitors; T lymphocyte stimulants | Subcutaneous |
| Sirexatamab (DKN-01) | Leap Therapeutics | II | DKK1 protein inhibitors | Intravenous |
| NP137 | NETRIS Pharma | II | Antibody-dependent cell cytotoxicity; T lymphocyte stimulants | Intravenous |
| COM 701 | Compugen | I/II | Antibody-dependent cell cytotoxicity; CD112 receptor antagonists; T lymphocyte stimulants | Intravenous |
| XMT-1660 | Mersana Therapeutics | I | Tubulin inhibitors | Intravenous |
Learn more about the emerging endometrial cancer therapies @ Endometrial Cancer Clinical Trials
Endometrial Cancer Therapeutics Assessment
The endometrial cancer pipeline report proffers an integral view of the emerging endometrial cancer therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Endometrial Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Exportin 1 protein inhibitors, Cyclin-dependent kinase 4 inhibitors, Cyclin-dependent kinase 6 inhibitors, Antibody-dependent cell cytotoxicity, T lymphocyte stimulants, Programmed cell death-1 ligand-1 inhibitors, Endopeptidase Clp stimulants, TNF-related apoptosis-inducing ligand receptor agonists, DKK1 protein inhibitors, Tubulin inhibitors, Epidermal growth factor receptor antagonists
- Key Endometrial Cancer Companies: Eli Lilly and Company, Karyopharm Therapeutics, NETRIS Pharma, TORL Biotherapeutics, Compugen, 3D Medicines, Evergreen Therapeutics, Acrivon Therapeutics, Eisai, Xadcera Biopharmaceutical (Suzhou) Co., Ltd., Huabo Biopharm Co., Ltd., Multitude Therapeutics, Context Therapeutics, Leap Therapeutics, Mersana Therapeutics, MacroGenics, Accent Therapeutics, Shanghai Henlius Biotech, Imva,x and others.
- Key Endometrial Cancer Pipeline Therapies: Selinexor, Abemaciclib, NP137, TORL-1-23, COM 701, Envafolimab, EG-007, Prexasertib, E7386, DM002, HB0025, AMT-151, CTIM-76, Sirexatamab (DKN-01), XMT-1660, MGC026, DHX9 (ATX-559), HLX17, IEC-001, and others.
Dive deep into rich insights for new endometrial cancer treatments, visit @ Endometrial Cancer Drugs
Table of Contents
| 1. | Endometrial Cancer Pipeline Report Introduction |
| 2. | Endometrial Cancer Pipeline Report Executive Summary |
| 3. | Endometrial Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Endometrial Cancer Clinical Trial Therapeutics |
| 6. | Endometrial Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Endometrial Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Endometrial Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Endometrial Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Endometrial Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Endometrial Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Endometrial Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the endometrial cancer pipeline therapeutics, reach out @ Endometrial Cancer Therapeutics
Related Reports
Endometrial Cancer Epidemiology Forecast
Endometrial Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted endometrial cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Endometrial Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key endometrial cancer companies, including GlaxoSmithKline, Merck & Co, AstraZeneca, Karyopharm Therapeutics, Evergreen Therapeutics, Incyte Corporation, among others.
Advanced Endometrial Cancer Pipeline
Advanced Endometrial Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key advanced endometrial cancer companies, including Incyte Corporation, Byondis B.V., Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Ability Pharmaceuticals SL, Zymeworks Inc., AstraZeneca, Eli Lilly and Company, Pfizer, Karyopharm Therapeutics, Genentech, Eli Lilly and Company, Genentech, Inc., NETRIS Pharma, Five Prime Therapeutics, Inc., Millennium Pharmaceuticals, Inc., Novartis Oncology, Takeda, Mundipharma-EDO GmbH, Zai Lab (Shanghai) Co., Ltd., Haihe Biopharma Co., Ltd., Xencor, Compugen Ltd, Checkpoint Therapeutics, Inc., Celon Pharma SA, Dragonfly Therapeutics, among others.
Endometriosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key endometriosis companies, including Enteris BioPharma, Bayer, Hope Medicine, Tiumbio, Organon, among others.
Endometriosis Pain Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key endometriosis pain companies, including Ferring Pharmaceuticals, ObsEva SA, Myovant Sciences, Hope Medicine (Nanjing) Co., Ltd, among others.
Endometriosis Pain Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key endometriosis pain companies, including Ferring Pharmaceuticals, ObsEva SA, Myovant Sciences, Hope Medicine (Nanjing) Co., Ltd, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
